juxinde@used-equipments.com +86 13992560725
- All
- Product Name
- Product Keyword
- Product Model
- Product Summary
- Product Description
- Multi Field Search
Views: 0 Author: Site Editor Publish Time: 2023-05-11 Origin: Site
Calcination is the thermal treatment of solid compounds, such as mixed carbonate ores, whereby the compound is raised to high temperatures without melting with a limited supply of ambient oxygen (i.e. the gaseous O2 portion of the air), usually to remove impurities or volatile substances and/or cause thermal decomposition.The root of the word calcination refers to its most prominent use, the removal of carbon from limestone (calcium carbonate) by burning to produce calcium oxide (quicklime).This calcination reaction is CaCO3(s) → CaO(s) + CO2(g). Calcium oxide is an important ingredient in modern cement and is also used as a chemical flux in smelting. Industrial calcination often emits carbon dioxide (CO2), making it a major contributor to climate change.Calciners are steel cylinders that rotate in a furnace and undergo indirect high-temperature treatment (550–1150 °C, or 1000–2100 °F) in a controlled atmosphere.
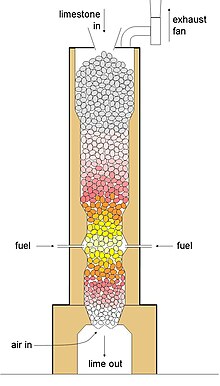
The calcination process gets its name from the Latin calcinare (to burn lime) because its most common application is to break down calcium carbonate (limestone) into calcium oxide (lime) and carbon dioxide to make cement.The products of calcination are often collectively referred to as "calcin", regardless of the actual mineral being heat-treated.Calcination is carried out in furnaces or reactors (sometimes called kilns or calciners) of various designs, including shaft furnaces, rotary kilns,multiple hearth furnaces and fluidized bed reactors.
Examples of calcination processes include:
Decomposition of carbonate ores, such as calcination of limestone to remove carbon dioxide;
Decomposition of hydrated minerals, such as removal of water of crystallization in the form of water vapor in the calcination of bauxite and gypsum, carbonate ores;
Decomposes volatile substances in raw petroleum coke;
Heat treatments that affect phase transitions such as anatase to rutile or devitrification of glassy materials;
Removal of ammonium ions in zeolite synthesis;
Defluorination of uranium fluoride to uranium dioxide and hydrofluoric acid gas.
Reactions
Calcination reactions usually occur at or above the thermal decomposition temperature (for decomposition and volatilization reactions) or transition temperature (for phase transition).This temperature is usually defined as the temperature at which the standard Gibbs free energy of a particular calcination reaction is equal to zero.